Abstract
Introduction. The randomized GALLIUM trial showed that Obinutuzumab-based immunochemotherapy and maintenance achieves a longer progression-free survival (PFS) and a lower rate of early disease progression at 24 months (POD24) than rituximab-based therapy. These therapeutic benefits were not yet established in a real-life setting. We present early results of the first study worldwide designed to assess the effectiveness and safety of obinutuzumab plus chemotherapy in patients with previously untreated advanced follicular lymphoma (FL) and a FLIPI score ≥2.
Methods. URBAN [NCT04034056] is a non-interventional, retrospective/prospective study involving 46 sites in Italy. The treatment plan included an obinutuzumab-chemotherapy (CHOP, CVP or Bendamustine) induction phase (6 months), followed by bimonthly obinutuzumab maintenance (24 months), and 1-year follow-up period after the last obinutuzumab administration. Participants should have received at least 2 cycles of obinutuzumab-chemotherapy induction before the enrolment (retrospective part). Treatment was delivered according to existing regulatory labels and local reimbursement requirements. The primary study endpoint was POD24 rate, i.e. progression of disease or death due to progression within 24 months from treatment initiation. Secondary endpoints were 2-year PFS and tumor response at end of induction (EOI). We report POD24 and 2-year PFS results for patients who started treatment 24 months before the clinical cut-off date (CCOD) of 31-Jan-2022 and the response at EOI for those who completed induction at CCOD. Patients with/without POD and PFS at 24 months were compared by gender, age, time from diagnosis to treatment start, FLIPI, ECOG performance status, and chemotherapy backbone.
Results. At CCOD, 299 patients have been enrolled, 294 were at the EOI, and 178 and 189 patients were evaluable at 24 months for POD24 and PFS, respectively. Enrolled patients (n=299) had a median age of 62 years, with 158 (52.8%) females and 182 patients (59.9%) with a high (≥3) FLIPI score. The median time from diagnosis to treatment was 1.6 (0-71.5) months.
Among the 294 patients who completed the induction, 263 were assessed for response at EOI. Response was assessed by PET-CT in 207 patients, by CT scan only in 40 and by other techniques in 16. In patients assessed with PET-CT, the overall response rate (ORR) was 97.6%, with complete response (CR) in 175 patients (84.5%), partial response (PR) in 27 (13.0%), stable disease (SD) in 2 (1.0%) and progressive disease (PD) in 2 (1.0%) (1 patient was not evaluable, N.E.); in patients assessed by CT scan only, ORR was 90%, with CR in 24 patients (60.0%), PR in 12 (30.0%), and PD in 3 (7.5%) (1 patient N.E.).
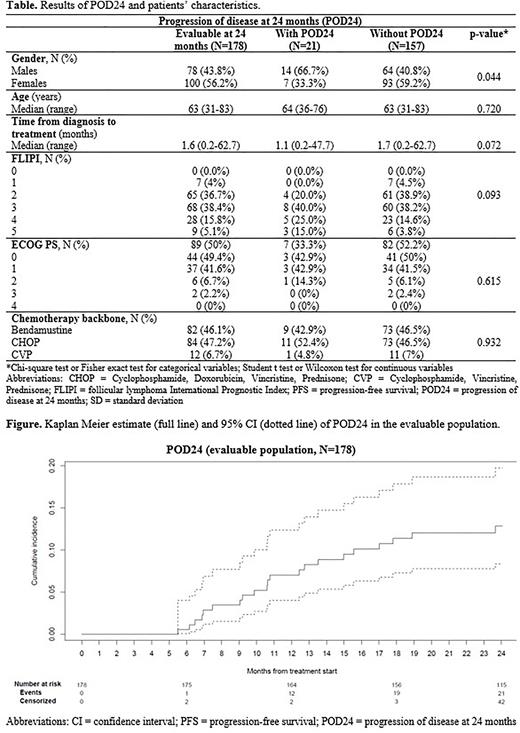
The figure shows the Kaplan-Meier estimates for POD24. The results of POD24 by patients' characteristics, including the comparisons between patients with and without POD at 24 months, are summarized in the Table. It is important to note that due to the COVID-19 pandemic, 16.4% and 16.8% of patients respectively evaluable for POD24 and 2-year PFS had not received the planned obinutuzumab maintenance. At the time of CCOD, 21 (11.8%) patients had a POD24 event and 157 (88.2%) did not. The rate of patients with POD24 was significantly higher in males than in females (p=0.044), but no statistically significant differences were observed for other variables. Due to study timeline, all POD24 events occurred during the pandemic.
Among the 189 patients evaluable for PFS, we observed a 2-year PFS of 83.1%. No statistically significant correlations have been identified between PFS events and clinical characteristics.
No new or unexpected safety signals were reported. Most common any grade adverse events were neutropenia (22.1% of patients retrospectively and 26.1% of patients prospectively), thrombocytopenia (9.7% and 5.1%, respectively) and infections, including COVID-19 (8.4% and 27.1%, respectively).
Conclusions. The results of this interim analysis showed that treatment of previously untreated FL patients with obinutuzumab and chemotherapy was associated with low POD24 rates, high rates of complete response and a substantial 2-year PFS. Despite the different patient populations and the likely impact of COVID-19 pandemic on the overall management of accrued patients, these real-life results are in line with those from the pivotal GALLIUM trial.
Disclosures
Pinto:F. Hoffmann-La Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck Sharp and Dohme: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte Italy: Membership on an entity's Board of Directors or advisory committees; Servier Affaires Medicales: Speakers Bureau. Guardalben:Roche S.p.A.: Current Employment. Caltagirone:Roche S.pA.: Current Employment, Current holder of stock options in a privately-held company. Piparo:Roche S.pA.: Current Employment, Current holder of stock options in a privately-held company. Patti:Takeda: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Abbvie: Consultancy, Other: Travel, accommodations. Puccini:Beigene: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Hohaus:Incyte: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Stefani:Roche: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Kiowa: Speakers Bureau; Gentili: Speakers Bureau. Zilioli:Takeda: Membership on an entity's Board of Directors or advisory committees, Other: travel expenses, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees; Gentili: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Janssen: Other: travel expenses, Speakers Bureau; Roche: Consultancy. Arcaini:Celgene/Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees; Verastem: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Research Funding. Gritti:Italfarmaco: Membership on an entity's Board of Directors or advisory committees; Clinigen: Consultancy; Beigene: Consultancy; Incyte: Consultancy; Genmab: Membership on an entity's Board of Directors or advisory committees; Sandoz: Other: Support for attending meetings; Ideogen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite-Gilead: Membership on an entity's Board of Directors or advisory committees; IQVIA: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings. Ladetto:GSKI, Gentili, Sandoz: Honoraria; Celgene: Honoraria, Research Funding; Roche, Eusapharma, Takeda, Regeneron: Honoraria; Gilead/Kite, Novartis: Honoraria; Regeneron, Incyte, Jazz: Honoraria; ADC Therapeutics: Honoraria, Research Funding; BeiGene: Honoraria, Research Funding; Acerta, Amgen: Honoraria; ADC Therapeutics: Honoraria, Research Funding; AbbVie: Honoraria; Jansenn: Honoraria, Research Funding. Zinzani:Secura Bio: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Eusapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; University of Bologna: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal